The PCQI course participants often showed a particular interest in the topic of validation, when and how to validate, what is acceptable and what is not, with regards to compliance with the FDA requirements.
The Preventive Controls for Human Food requirements stipulate in 21 CFR 117.160:
(a) You must validate that the preventive controls identified and implemented in accordance with § 117.135 are adequate to control the hazard as appropriate to the nature of the preventive control and its role in the facility’s food safety system.
(b) The validation of the preventive controls must be performed (or overseen) by a preventive controls qualified individual.
According to § 117.3 Definitions, validation means obtaining and evaluating scientific and technical evidence that a control measure, combination of control measures, or the food safety plan as a whole, when properly implemented, is capable of effectively controlling the identified hazards.
While the Preventive Controls for Human rule provided flexibility in justifying the reasons not to validate sanitation preventive controls, allergen preventive controls, and others – except in certain conditions (to be discussed in the next post), validation of process controls is a must-to-do.
This decision tree , published by Ceylan et al. 2021 in Comprehensive Reviews in Food Science and Food Safety, is a practical and simple tool to use in supporting the decision of when and which validation study approach is most applicable.
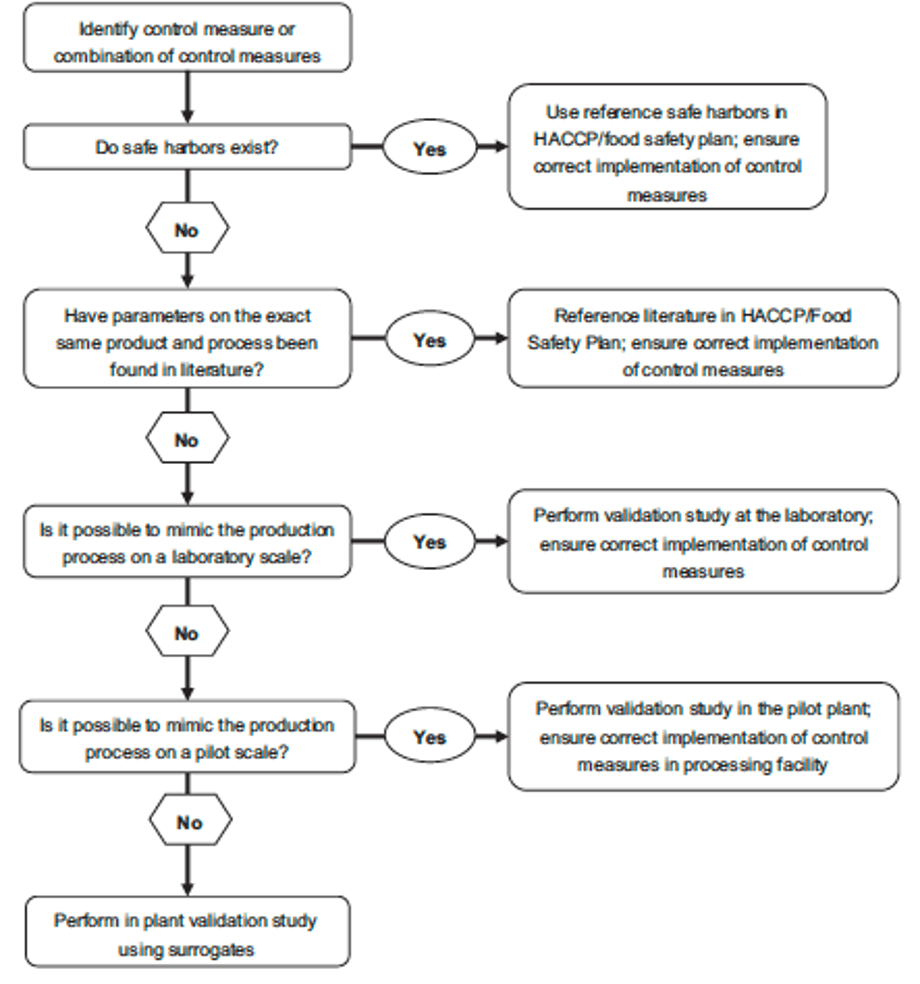
You find the decision tree in the link provided above to access the full article. If you find it useful, why not sharing it with you team members to enhance their knowledge on the importance of science-based approach in food safety.
Got questions on your FSP and risk assessment, or on regulatory matters? I will be happy to hear from you.